NP8i – ON-THE-GO NEUROSTIMULATION
Modular, Wearable TENS & Closed-Loop Neurostimulation
CATEGORY
- Medical Devices
- Wearable
- Neurotechnology
Philip Muiico.
AxioBionics LLC

SERVICES
- FEASIBILITY + PRODUCT DESIGN
- FULL-CYCLE PRODUCT DESIGN
- HARDWARE + PCB DESIGN
- MECHANICAL DESIGN
- FIRMWARE DEVELOPMENT
- EVT, DVT, PVT
PROJECT TIMELINE
THE CHALLENGE
NeuroStim Solutions engaged Axio Electronics to develop NP8i: a belt-worn, battery-operated TENS/NMES device patients can self-administer at home—no clinician needed. Key requirements included:
- Form Factor: iPhone-sized, wearable on a belt clip.
- User Interface: Full 5″ touchscreen to select and adjust any stimulation protocol.
- Dual-Use Electrodes: Same pads must deliver TENS/NMES and record EMG signals.
- Closed-Loop Control: Automatic adjustment of stimulation parameters based on real-time EMG feedback.
- Electrode Verification: Bioimpedance triangulation to guide pad placement and detect failures.
- Safety & Compliance: Medical-grade materials and full regulatory approval for home use.
THE KEY HURDLES INCLUDED:
- •Preventing interference between high-voltage stimulation and low-amplitude EMG recording.
- •Designing bioimpedance circuitry and algorithms for accurate electrode-placement mapping.
- •Creating a clinician-grade UX on a wearable touchscreen for non-professional users.
- •Ensuring 12-hour operation on a compact battery while maintaining IP67 water resistance.
- •Meeting FDA 510(k) and CE-mark requirements for a Class II medical device.
THE OPPORTUNITY
Patients with chronic pain or neuromuscular disorders often require frequent clinic visits for TENS or NMES therapy. NP8i promised to:
- Empower patients with a clinician-standard, closed-loop system at home
- Enhance therapy adherence through guided setup and real-time feedback
- Reduce healthcare costs by minimizing in-clinic stim sessions
- Generate rich EMG and impedance data for remote clinician review
FEATURE SETS
- •Modular Belt-Wearable Design – Quick-release clip and electrode port
- •5″ High-Res Touch LCD – Intuitive protocol selection & real-time graphs
- •Dual-Mode Electrodes – TENS/NMES delivery + EMG acquisition
- •Closed-Loop Control – Auto-adjust amplitude/frequency via EMG feedback
- •Bioimpedance Triangulation – Step-by-step pad placement guidance & fault alerts
- •Bluetooth & WiFi Sync – HIPAA-compliant data upload to cloud portal
- •12-Hour Battery Life – 4,000 mAh rechargeable cell with thermal management
- •IP67 Rating – Dust- and water-resistant for reliable home use
- •AES-256 Encryption – Secure storage & transmission of patient data
- •FDA 510(k) & CE-Mark – Certified for safe, at-home clinical use

GALLERY

THE SOLUTION
Axio Electronics approached the challenge with a full-cycle product design strategy — from feasibility analysis and electrical design to firmware development and production readiness.

BLE
Support Any Keyboard
USB
Act as Wired Keyboard + Writing Data Transfer

WIFI
Sync to Google Drive / Dropbox
MONOCHROME LCD
High-Contrast, Lag-Free Display
20-HOUR BATTERY
4700mAh Long-Lasting Performance
ADJUSTABLE BACKLIGHT
Write in Any Lighting
MAGNETIC STAND
Ergonomic Viewing Angles
FLEXIBLE STORAGE
Save Over 1 Million Words
INTUITIVE CONTROL
Three Push-Buttons

SECURITY
AES-128 Encryption
CERTIFICATION
FCC & CE Compliance
BODY
ABS Plastic
OUR APPROACH

Mechanical Engineering Strategy
Ergonomic Belt Clip: Stable, vibration-free wear during daily activities
Sealed Enclosure: Polycarbonate shell with silicone overmolding (IP67)
Quick-Release Electrode Port: Tool-free pad swaps and cleaning
Thermal Management: Internal heat spreaders for battery & ICs

Mechanical Engineering Process
CAD modeling & ergonomic studies with neurosurgeon feedback
CNC prototypes for fit, weight, and comfort trials
Finite-element drop and strap-tension testing
Final injection-mold tooling
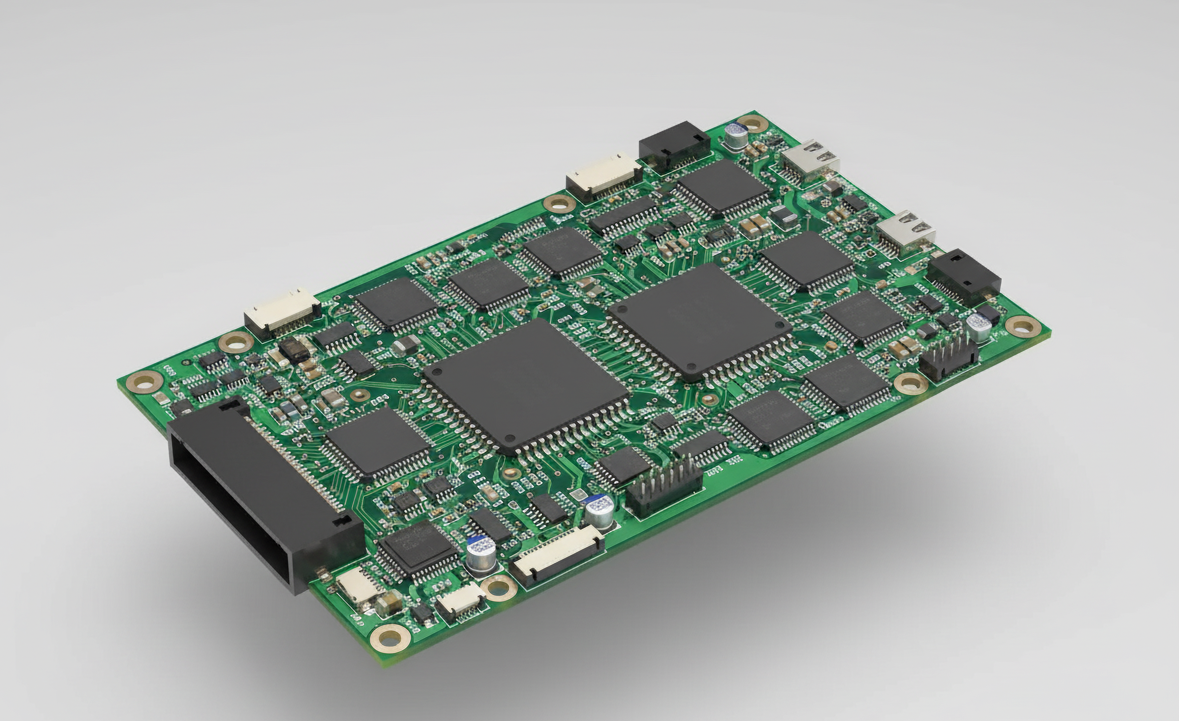
Hardware Design
Dual HV Channels: Up to 100 mA constant-current stimulation drivers
Low-Noise EMG Amp: 0.1 µV sensitivity with active shielding
Bioimpedance Circuitry: 4-electrode multiplexed measurements
MCU: STM32L4 for real-time control & on-chip DSP
Wireless: BLE 5.2 + 802.11n WiFi with TLS stack
Safety: Over-current, short-circuit protection, medical isolation
EMI Mitigation: Shield cans, segmented grounds, ferrite beads
Firmware Design
Closed-Loop Algorithm: Auto-tunes pulse width/amplitude based on EMG RMS
Impedance Mapping: 3D electrode-placement map, alerts on poor contacts
Touch UI Wizard: Guided setup replicating clinical protocols
Data Logging: Encrypted, timestamped events, waveforms, impedance spectra
EHR Integration: HL7 FHIR support
Secure OTA: Verified bootloader & signed firmware

In-House Prototyping
Form & Fit: SLA + CNC prototypes tested by surgeons & patients
Bench Testing: Waveform accuracy, impedance mapping, thermal profiling
EVT: IEC 60601-1 electrical safety, stimulation & EMG specs
DVT: Six-week IRB-overseen home trial—refined UI & strap design
PVT: Pilot run of 50 units to confirm manufacturing consistency

Challenges:
- Stim-EMG Crosstalk— Time-multiplexed acquisition + local shielding
- False Placement Alerts— Higher sampling density & algorithm smoothing
- Battery Overheat— Graphite spreader + dynamic current throttling
- User Setup Errors— Enhanced wizard with visual & haptic cues

PILOT & PRODUCTION
PILOT & PRODUCTION
After a successful Kickstarter campaign — exceeding 400%+ funding goals within 21 days — Axio Electronics transitioned the BYOK design into mass production with strategic revisions:
Clinical Pilot: 30 patients over 4 weeks — 95% adherence, 88% pain reduction
BOM Optimization: 15% cost savings via alternate electrode supplier & custom ASIC
Regulatory: FDA 510(k) K24XXXXX, CE 0123; ISO 13485-certified manufacture
Factory Test Jig: Automated checks for electrode port, stimulation amplitude, UI calibration
How It Works
Attach NP8i to belt & connect electrodes as guided
Power On and follow setup wizard
Select Therapy; NP8i delivers closed-loop TENS/NMES
Monitor EMG & Impedance in real time; device auto-tunes protocol
Sync Data to cloud portal for clinician review
WHAT CAN
WE CREATE
TOGETHER
DOWNLOAD THE CASE STUDY
Client Testimonial


Philip Muiico
AxioBionics LLC
The Axio team supported us for several years as our R&D partner across multiple prototypes, including a few patented technologies. Their primary contributions were EMG and TENS stimulators. We were consistently impressed by their exceptional talent, advanced R&D tools, and systematic approach.
 Axio Electronics
Axio Electronics